What Is Static Electricity
Static electricity is an everyday phenomenon that surprises us with a small shock or makes our hair stand on end. Although it may seem like magic, its origin lies in the behavior of subatomic particles. In this article, you’ll discover how the exchange of electrons between materials gives rise to this curious effect—and why it’s more common in winter.
22 de mayo de 2024

All the objects we see are made up of tiny particles called atoms. Although the word atom means “indivisible,” atoms are actually made up of even smaller particles. When the atom was first named, we didn’t know this. The particles that make up atoms are called protons, neutrons, and electrons, and they are very different from each other. One key difference is their electrical charge: protons have a positive charge, electrons have a negative charge, and neutrons have no electrical charge; they are neutral.

Atom and their particles.
Atoms usually have the same number of protons and electrons, so the positive and negative charges cancel each other out. That’s why atoms are usually electrically neutral. But if we rub two objects against each other, some electrons can move from one atom to another. Atoms that gain new electrons take on a negative charge, while those that lose electrons become positively charged. When charges are separated like this, it’s called static electricity.
Static electricity is an imbalance of positive and negative charges
If two things have opposite charges, they attract each other. If they have like charges, they repel each other. This explains why your hair stands on end when you take off a sweater or a wool hat. When the hat rubs against your hair, some electrons move from your hair to the wool. As a result, each strand of hair becomes positively charged. Since things with the same charge repel one another, the hairs try to move away from each other. But because they’re tightly rooted in your scalp (luckily!), the best they can do is stand up and spread apart.
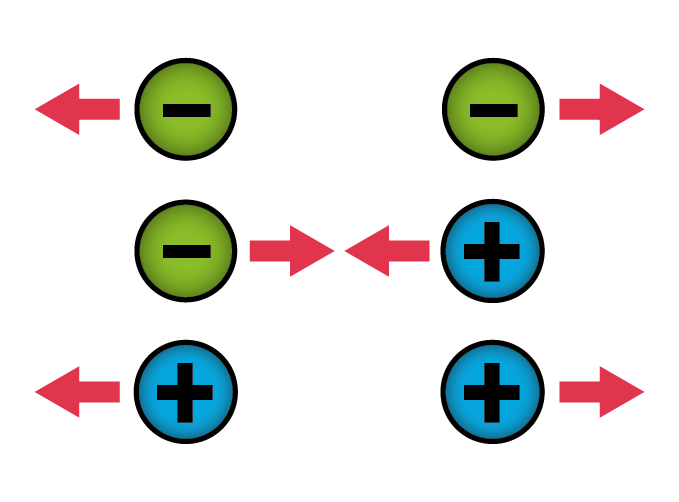
Atraction and repulsion of two objects.
When you walk across a rug—especially a synthetic one—you’re rubbing against it, and some of its electrons move to you. Then, when you touch a doorknob… ZAP! The electrons move from you to the doorknob, and you feel a static shock because your nerve receptors are stimulated.
The more you rub two objects together, the more electrons move—and the larger the charge that builds up. Scientists believe that it is not friction itself that causes the electrons to move, but rather the contact between the two materials.
Friction just increases the surface area where contact happens.
When we rub two materials together, electrons from one of them pass to the other.
Which of the two materials releases electrons and which one receives them?
Scientists have classified materials based on how easily they hold onto or release electrons. This classification is called the triboelectric series. Here’s a list of some common materials:
Under ideal conditions, if two of these materials rub together, the one higher on the list gives up electrons and becomes positively charged. You can test this for yourself by experimenting with them.

Charge is not created or destroyed
It might seem like when we charge an object with static electricity, we’re creating a kind of “new” energy out of nowhere. But that’s not what’s happening. What actually happens is that electrons are simply moving from one place to another. This idea is called the law of conservation of electric charge, and it’s one of the fundamental laws in physics.
Imagine you’re playing a game where you pass marbles from one pocket to another. You’re not making new marbles—you’re just moving around the ones you already had. The same thing happens with electrons. If an object has a positive charge, it’s because it lost some electrons. And if another object has a negative charge, it’s because it gained them. The total charge in the system stays the same; it just gets redistributed.
This principle is really important in science because it helps us understand that matter and energy are conserved. We can’t just make electrons appear when we want to, and we can’t make them disappear either. In all electrical processes—from everyday static shocks to the complex reactions in your body or in a thunderstorm—the total amount of electric charge remains constant.
This concept also applies to things like electric circuits, batteries, and even processes like electrolysis or photosynthesis. Static electricity might seem like just a fun trick, but it follows the same rules that govern more complex phenomena.
Why is static electricity more common in winter than in summer?
You’ve likely noticed that shocks and frizzy hair happen more often in winter. The key lies in the humidity of the air. In winter, the air tends to be drier, especially indoors where we use heating. And dry air is a poor conductor of electricity. That means electric charges stick around longer on objects… and on our bodies!
In contrast, during the summer—or in more humid places—the water vapor in the air makes it easier for electric charges to spread out and disappear. Water vapor acts like a kind of “bridge” that lets electrons slowly escape without us even noticing. Because of this, static electricity doesn’t build up as much, and we’re less likely to feel a shock.
That’s why on cold, dry days, things like rubber-soled shoes, synthetic carpets, and wool sweaters make the perfect combo for creating static electricity. In fact, some schools and science labs use humidifiers in winter to prevent sparks that might damage sensitive electronic equipment.
What about lightning?
Lightning might seem like a far-off and dangerous event, but it’s actually just a giant version of static electricity. Something very similar happens when you walk across a carpet and then touch a doorknob—just on a much bigger scale!
Inside storm clouds, wind and air movements make water droplets and ice crystals crash and rub against each other. This constant, chaotic motion causes a separation of charges: some parts of the cloud become positively charged, while others become negatively charged. Usually, the bottom of the cloud builds up a negative charge.

As this negative charge increases, it starts pulling positive charges up from the ground—even from trees, buildings, or people. When the difference between the two charges gets large enough, there’s a sudden flow of electrons: that’s lightning. The electrons move from the cloud to the ground (or sometimes from cloud to cloud), releasing a huge amount of energy in the form of light, heat, and sound.











Leave a Reply